HeartLung AI-CVD® Investigator Dr. Reza Mirjalili Wins SCCT 2026 Rapid-Fire Award for AI-CVD® Heart Failure Prediction
Award-winning presentation recognizes AI-CVD-HF heart failure prediction model leveraging coronary artery calcium (CAC) scans
HONG KONG, January 27, 2026 /EINPresswire.com/ -- HeartLung AI announced today that Seyed Reza Mirjalili, MD, a fellow AI-CVD® investigator, has been named the Rapid-Fire Winner at SCCT Global 2026 in Hong Kong, recognizing his outstanding scientific presentation and contribution to the field of preventive cardiovascular CT.Dr. Mirjalili received the award for his rapid-fire abstract titled: “AI-CVD-HF: A novel heart failure prediction model based solely on coronary artery calcium scans outperforms PREVENT-HF.” The work highlights the potential of AI-enabled quantitative imaging to strengthen heart failure risk prediction using routinely acquired CT data, supporting earlier identification of patients who may benefit from proactive prevention strategies.
The AI-CVD-HF study presents a deep-learning approach that uses routinely acquired non-contrast coronary artery calcium (CAC) CT scans, together with standard clinical risk factors, to improve prediction of future heart failure risk and benchmark performance against the PREVENT-HF model, the current standard of care. The platform automatically extracts a comprehensive set of quantitative biomarkers from the same CAC CT exam, including coronary calcium and other cardiovascular calcifications, cardiac chamber measurements, aortic and pulmonary artery dimensions, body composition metrics (visceral/subcutaneous/pericardial fat and muscle density), liver fat, lung density patterns suggestive of emphysematous/interstitial changes, and opportunistic vertebral bone mineral density with derived T- and Z-scores. The analysis was evaluated across large, well-characterized cohorts (including MESA and the Framingham Heart Study Offspring MDCT sub-study) with subgroup assessments by age, sex, race/ethnicity, diabetes, and hypertension, and included comparison of 10-year risk categorization between AI-CVD-HF and PREVENT-HF. In summary, AI-CVD-HF outperformed the current standard-of-care risk model, PREVENT-HF, providing stronger discrimination and more informative risk stratification for future heart failure using data available from routine CAC CT imaging. This supports a more scalable, opportunistic approach to identifying higher-risk patients earlier—without requiring additional testing or changes to clinical workflow.
“Dr. Mirjalili’s Rapid-Fire win at SCCT Global is a major recognition of both scientific rigor and clinical relevance,” said Dr. Morteza Naghavi, CEO of HeartLung. “We are proud to commend Dr. Mirjalili and the entire AI-CVD team at HeartLung for advancing practical, scalable innovation that helps clinicians identify hidden cardiovascular risk earlier and more effectively.”
This recognition follows HeartLung’s recent U.S. FDA 510(k) clearance of AI-CVD®, the broadest opportunistic cardiovascular & multisystem CT screening platform ever authorized, further validating the clinical value and readiness of HeartLung’s AI-driven approach to CVD prevention.
About HeartLung.AI
HeartLung Corporation is a medical technology company dedicated to advancing AI-enabled, CT-based opportunistic screening and early disease detection. HeartLung’s mission is to shift healthcare from late-stage disease treatment to earlier identification and prevention, using artificial intelligence to unlock clinically actionable information embedded within routine medical imaging.
HeartLung develops FDA-cleared AI technologies for the opportunistic detection and prevention of cardiovascular disease, lung cancer, emphysema/COPD, osteoporosis, myosteatosis, fatty liver disease, and other life-threatening conditions—often years before symptoms appear.
The company has received FDA Breakthrough Device Designation and FDA 510(k) clearance for AutoChamber™, an AI-powered tool that detects enlarged cardiac chambers and left ventricular hypertrophy on non-contrast chest CT scans, including low-dose CT used for lung cancer screening and contrast-enhanced coronary CT angiography (CCTA). HeartLung has also obtained FDA 510(k) clearance for AutoBMD™, the only CT-based, DEXA-equivalent opportunistic osteoporosis screening technology cleared by the FDA and reimbursed by Medicare.
These technologies are now integrated within AI-CVD®, HeartLung’s flagship FDA-cleared platform for large-scale opportunistic screening across cardiovascular and multisystem disease domains. By enabling clinicians to extract far greater preventive value from CT scans that are already being performed, HeartLung aims to redefine how imaging contributes to population health, value-based care, and early disease prevention.
For more information, visit https://www.heartlung.ai
About AI-CVD®
The U.S. Food and Drug Administration (FDA) has approved the following Indications for Use for AI-CVD®:
AI-CVD® is an opportunistic, AI-powered quantitative imaging tool that provides automated CT-derived anatomical and density-based measurements for clinician review. Using AI-CVD® quantitative imaging measurements and clinical evaluation, healthcare providers can investigate patients who are unaware of their risk of:
• Coronary heart disease
• Heart failure
• Atrial fibrillation
• Stroke
• Osteoporosis
• Liver steatosis
• Diabetes
• Other adverse health conditions that may warrant follow-up
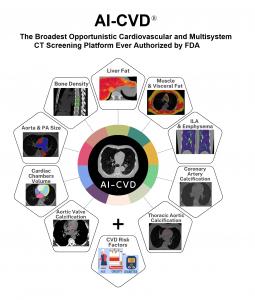
Ten FDA-Cleared Opportunistic Measurement Domains in a Single Platform
AI-CVD® includes FDA-cleared modules for:
• Coronary artery calcium (CAC) scoring
• Aortic wall calcium
• Aortic valve calcium
• Mitral valve calcium
• Cardiac chamber volumetry
• Epicardial fat volumetry
• Aorta and pulmonary artery sizing
• Lung attenuation analysis
• Liver attenuation analysis
• Bone mineral density and muscle–fat composition
Built as a modular, AI-powered quantitative imaging platform, AI-CVD® automatically extracts clinically relevant anatomical and density-based measurements from existing chest and abdominal CT scans—without additional imaging, radiation, contrast, or workflow disruption.
AI-CVD® integrates multiple FDA-cleared technologies, including AutoChamber™ and AutoBMD™, into a unified system designed for opportunistic screening and early disease detection. The platform enables clinicians to identify patients who may warrant additional diagnostic testing, monitoring, or preventive action, using objective, CT-derived measurements from scans that are already being performed for other clinical indications.
Consistent with its FDA-cleared Indications for Use, AI-CVD® does not provide diagnostic interpretation or risk prediction. Instead, it equips healthcare providers with quantitative imaging insights that transform routine CT imaging into a scalable foundation for preventive care across cardiovascular, metabolic, pulmonary, and skeletal disease domains.
Learn more at https://www.heartlung.ai/aicvd
Marlon Montes
HeartLung Technologies
+1 310-510-6004
contact@heartlung.ai
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.